
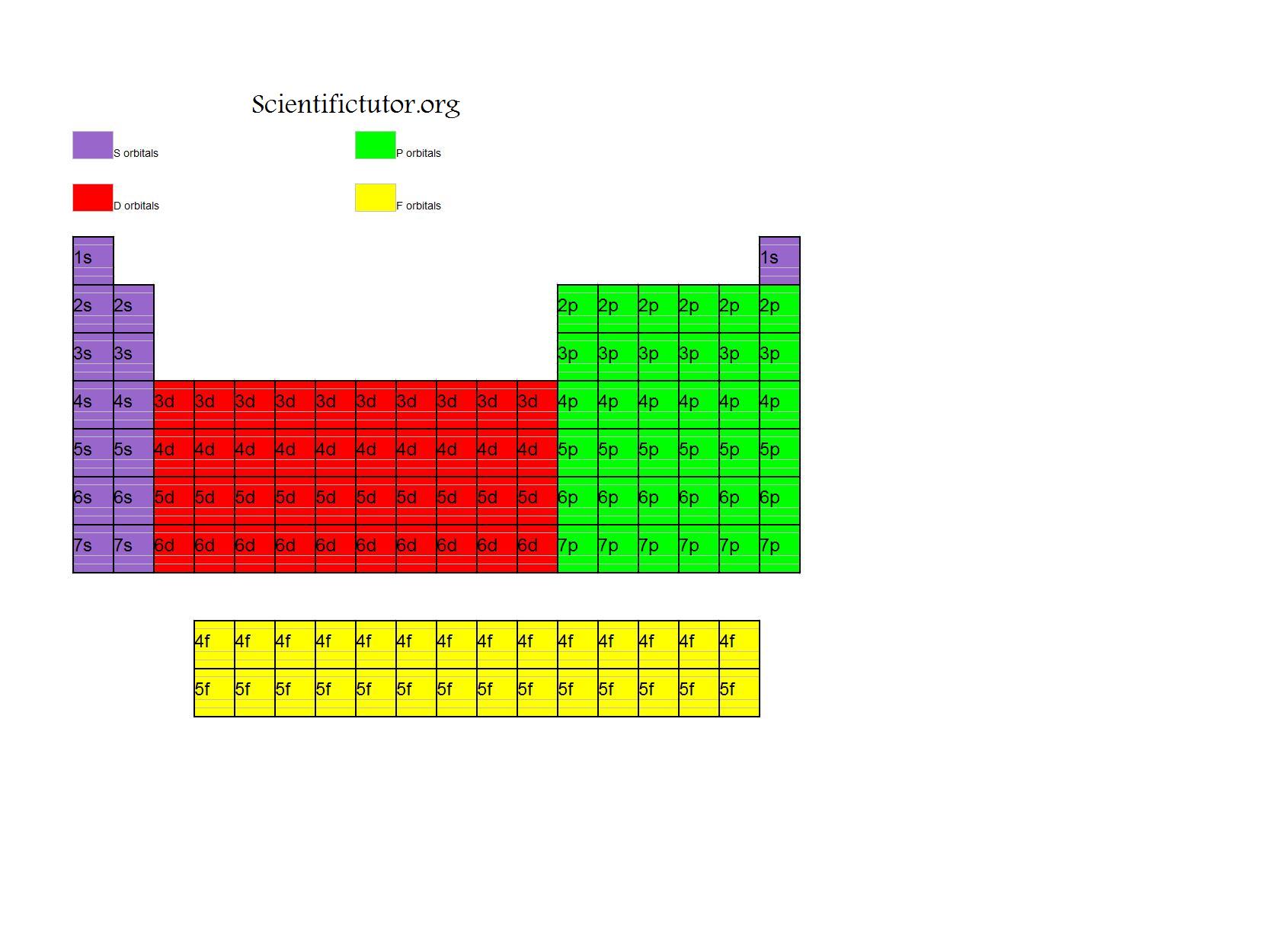
There is a shorthand way of writing electron configurations. The valence electron configurations for all of the elements are in the periodic table below. Iron has 8 valence electrons with 2 electrons in the 4s subshell and 6 electrons in the 3d subshell. Sodium, in Group 1A, has 1 valence electron in the 3s oribital and chlorine in Group 7A has 7 valence electrons, with 2 electrons in the 3s and 5 electrons in the 3p orbitals. For transition elements, the ns and the (n – 1)d electrons are the valence shell electrons. For the main group elements, the number of valence shell electrons are equal to the group number (Groups 1A to 8A). The electrons in the valence shell are the valence electrons. The outermost shell is the valence shell. Note, when the 4f orbitals were filled, we then fill the 5d orbitals and the remaining electrons go into the 6p orbitals. Recall when we fill the 6s orbital, the next orbitals to be filled are the 4f orbitals. The electron configuration for Sn is 1s 22s 22p 63s 23p 64s 23d 104p 65s 24d 105p 2. The electron configuration for Fe is 1s 22s 22p 63s 23p 64s 23d 6. The electron configuration is 1s 22s 22p 63s 23p 64s 23d 1. When reading the periodic table from left to right we fill all the way to the 4s orbital and then fill a 3d orbital with the last electron. Next, we write the electron configuration for Sc. In the figure below are the electron configurations for the first 20 elements. The orbital box diagrams are listed for the first 20 elements in the figure below. Boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin–one arrow pointing up and the other one pointing down.


An orbital box diagram can be written as well. The electron configuration for carbon is 1s 22s 22p 2. Reading the periodic table from left to right, the boron atom has 5 electrons, and the electron configuration is 1s 22s 22p 1. The four electrons of Be go into the 1s and the 2s orbitals giving the electron configuration of 1s 22s 2. Next we go to lithium with 3 electrons two of the electrons go into the 1s orbital and the third electron into the 2s orbital giving an electron configuration of 1s 22s 1. Helium has 2 electrons and both electrons go into the 1s orbital and the electron configuration is 1s 2. Hydrogen has 1 electron which goes into the 1s subshell. You only need to know where the blocks are located on the periodic table. When reading the periodic table from left to right, one can easily write an electron configuration without memorizing the filling order. The periodic table below, shows the s, p, d, and f-blocks. The filling order follows:ġs→2s→2p→3s→3p→4s→3d→4p→5s→4d→5p→6s→4f→5d→6p→7s→5f→6d→7pĮlectron configurations and orbital box diagrams can be written right from the periodic table. For example the 1s orbital is filled and then we can fill the 2s orbital and go on to the 2p orbitals. The lowest energy orbitals must be filled first. In other words, the orbitals in a subshell must be half filled before pairing electrons. Hund’s Rule specifies that when orbitals of equal energy are available, the lowest energy electron configuration has the maximum number of unpaired electrons with parallel spins. The Pauli Exclusion Principle states that each orbital may contain a maximum of 2 electrons electrons must have opposite spins.ģ. The Aufbau Principle states that electrons are always placed in the lowest energy sublevel that is available.Ģ.


 0 kommentar(er)
0 kommentar(er)
